Introduction
Our aim is to develop methods for reliable monitoring of local and systemic effects in patients undergoing orthopedic treatments or receiving implants.
Markers of early loosening
Bone markers such as Pro-C-terminal peptide of procollagen type I for bone formation, N-telopeptide of collagen type I for bone resorption, and osteoprotegerin, RANKL, and TRACP-5b for osteoclastic activity, are measured in patients with prosthetic joints to evaluate their clinical relevance in the diagnosis of early loosening. To recognize early signs of potential implant failure should be an invaluable diagnostic tool in patient monitoring.
Immune reactivity to prosthetic components
Since biomaterials may induce immunological reactions, including chronic inflammation, immunostimulation, autoimmunity, hypersensitivity, immunosuppression, the immune status of patients with prosthetic joints is evaluated. Moreover, potential hypersensitivity to prosthetic components is assessed by means of patch testing, i.e. analysis of various haptens present in orthopedic implants.
Histology of implant failure
Using a standardized protocol, the tissue reaction to wear debris in relation to the prosthesis design is currently analysed in order to identify the cause of implant failure.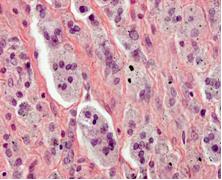
Toxicogenomics
In order to assess the amount of of metal ions systemically released in patients with metal joint prostheses, a systematic analysis is run using atomic absorption spectrophotometry.
