Referent
Pagination
Introduction
The research group focuses on In Silico Medicine, which involves the adoption of personalized computational models to support medical decisions and/or to assess the safety and efficacy of new medical products.
The activities mainly focus on applications related to the diagnosis and treatment of neuromusculoskeletal diseases, such as:
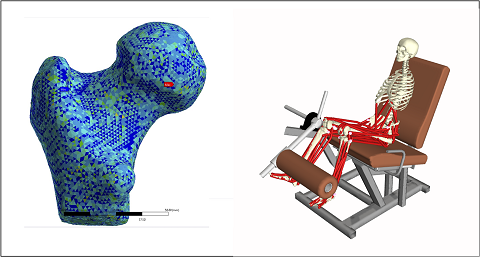
- Osteoarticular diseases: Finite element models are used to evaluate mechanical strength and risk of failure in bones (e.g., femur and vertebrae) under phisiological and/or phatological conditions (e.g., osteoporosis). Computational models are also used to predict and study implant failures (e.g., wear in hip and knee implants). Ongoing projects:
- BoneStrength (self-funded), aims to complete the development of the CT2S technology, extend its validation from the femur to the spine (including metastatic vertebrae), and qualify it with EMA
- IST4THR (self-funded), aims to develop in the long term a complete solution for in silico trials of total hip replacement medical devices
- COMPBIOMED (H2020), aims to scale up to HPC level in silico medicine applications, with a focus on MSK models
- Neuromuscular diseases: Computational models based on multibody dynamic systems are used to study the effect of musculoskeletal diseases (e.g., Parkinson and Sarcopenia) on motor control. Ongoing projects:
- MOBILISE-D (H2020), aims to qualify the average real-world walking speed, as measured with wearable sensors, as the main mobility biomarker in drug trials
- ForceLoss (RF2020), aims to develop, validate, and qualify subject-specific models of musculoskeletal dynamics as diagnostic and monitoring clinical tools.
Translation research is conducted on:
- Computational model verification and validation and regulatory science related to the adoption of these new technologies in clinical practice. Ongoing projects:
- STRITUVAD (H2020), aims to develop an in silico trial for tuberculosis therapies
- MOBILISE-D (H2020)
- Scalability and multiscale model orchestration. Ongoing projects:
- PRIMAGE (H2020), aims at developing a technology for the orchestration of in silico medicine models
- COMPBIOMED2 (H2020).
Also, different networking and community building initiatives are carried out to promote the wider adoption of in silico medicine. These include:
- InSilicoWorld, the first international online community for experts, researchers and professionals in the field of in silico medicine. The community hosts a discussion forum that collaboratively agreed on a set of Good Simulation Practices, besides other channels devoted to specific topics and project such as:
- Free Support Channel for computational medicine applications and scalability issues.
- Confidential forum where regulatory experts can freely discuss the best approaches to regulatory submission of In Silico Trial technologies for clinical practice
- International School on In Silico Trials, training initiative for decision-makers in medical industries, research hospitals, contract research organizations, regulatory agencies and notified bodies.

