Introduction
The mission of the Laboratorio di Tecnologia Medica (Medical Technology Laboratory) is to develop, to validate and to transfer to clinical practice every innovative technology that might improve the prevention, diagnosis, treatment, monitoring, or the rehabilitation of musculoskeletal diseases.
The lab has a staff of approximately 40 people including senior and junior researchers, as well as graduate and undergraduate students.
They are organised in five research units, each leaded by a Senior Researcher.
More info on our research outputs can be obtained from the description of the research units.
Research Activity
The Medical Physics group is carrying out research activities to improve diagnostic techniques applied to the orthopaedics, and to introduce Information and Communication Technology (ICT) to the clinical practice. The group has developed specific knowledge on microtomographic techniques to characterize bone tissue and biomaterials. Moreover, the group participates to national and international projects for the technology transfer of emerging technologies to the orthopaedic practice:
- European project VIRTUS: large scale diffusion of telemedicine services (2001-2003)
- Regional project co-financed by regione Emilia- Romagna HandHealth: for the validation of new handheld devices in the orthopaedic practice (2004-2006)
- National project co-financed by the Health Ministry: validation of computer aided medicine in orthopaedics, co-financed by the Health Ministry (2005-2007)
- European project LHDL: Living Human Digital Library: Interactive digital library services to access collections of complex biomedical data on the musculo-skeletal apparatus, (2006-2009)
- European project VPHOP: The Osteoporotic Virtual Physiological Human, future technologies for the prediction of risk of fractures in the osteoporotic patient (2008-2012)
- National project co-financed by the Health Ministry: Early diagnosis of pending failures of total hip arthroplasty with hard-to-hard bearings (2010-2014)
- National project co-financed by the Health Ministry: Manufacturing information of orthopedic articular prosthesis. Analysis of specific safety issues for radiotherapy, magnetic resonance, orthopedic surgery and identification of a traceability model (2012-2015)
- European Project MIMAS: Procedures allowing medical implant manufacturers to demonstrate compliance with MRI safety regulations (2018-2021)
- National project co-financed by the Health Ministry - ForceLoss: differential diagnosis of force loss in fragile elders (2021-2024
- European project In Silico World (ISW): Lowering barriers to ubiquitous adoption of In Silico Trials (2021-2024)
- National Plan for Complementary Investments to the PNRR - project DARE: Digital Lifelong Prevention (2022-2026)
- European project METASTRA: Computer-Aided effective fracture risk stratification of patients with vertebral metastases for personalised treatment through robust computational models validated in clinical settings (2023-2028)
X-ray microtomography (microCT) applied to bone investigation and biomaterials analysis
X-ray microcomputed tomography (microCT) is an emerging technique for the non-destructive characterization of small specimens, in the field of orthopaedics, dentistry and biomaterials research. It is based on the same basic principles as common computed tomography. The system installed at the LTM-IOR, (Skyscan 1072, Skyscan, Belgium, http://www.skyscan.be), offers high resolution imaging (5-20 microm/pixel) for specimens having sizes of about 4-20 mm. The system has built-in 2D and 3D image reconstruction capability.
Fields of investigation in the laboratory:
- bone histomorphometry (e.g. bone volume fraction, trabecular thickness)
- biomaterials characterization (e.g. porosity)
Characterization of the structural parameters together with the mechanical properties of the cancellous bone:
An advantage of microCT over conventional histology is that it is a non-destructive method of investigation, leaving the structure of the examined specimen intact and thus allowing further analysis, such as mechanical testing. This permits to combine the structural information of the bone specimen obtained by microCT (e.g. bone volume fraction, trabecular thickness) with the mechanical properties determined experimentally (e.g. elastic modulus, ultimate stress).
MicroCT examination of a human cancellous bone specimen before and after a mechanical compression test (specimen size: 10mm diameter, 20mm height):
Related Publications:
- Perilli E, Baruffaldi F, Bisi MC, Cristofolini L, Cappello A. A physical phantom for the calibration of three-dimensional X-ray microtomography examination. J Microsc. 2006 May;222(Pt 2):124-34
- Perilli E, Baruffaldi F, Visentin M, Bordini B, Traina F, Cappello A, Viceconti M. MicroCT examination of human bone specimens: effects of polymethylmethacrylate embedding on structural parameters. J Microsc. 2007 Feb;225(Pt 2):192-200
- Zauli G, Rimondi E, Stea S, Baruffaldi F, Stebel M, Zerbinati C, Corallini F, Secchiero P. TRAIL inhibits osteoclastic differentiation by counteracting RANKL-dependent p27Kip1 accumulation in pre-osteoclast precursors. J Cell Physiol. 2008 Jan;214(1):117-25
- Panaroni C, Gioia R, Lupi A, Besio R, Goldstein SA, Kreider J, Leikin S, Vera JC, Mertz EL, Perilli E, Baruffaldi F, Villa I, Farina A, Casasco M, Cetta G, Rossi A, Frattini A, Marini JC, Vezzoni P, Forlino A. In utero transplantation of adult bone marrow decreases perinatal lethality and rescues the bone phenotype in the knockin murine model for classical, dominant osteogenesis imperfecta. Blood. 2009 Jul 9;114(2):459-68
- Tassani S, Ohman C, Baleani M, Baruffaldi F, Viceconti M. Anisotropy and inhomogeneity of the trabecular structure can describe the mechanical strength of osteoarthritic cancellous bone. J Biomech. 2010 Apr 19;43(6):1160-6
- Tassani S, Ohman C, Baruffaldi F, Baleani M, Viceconti M. Volume to density relation in adult human bone tissue. J Biomech. 2011 Jan 4;44(1):103-8
- Giannini C, Siliqi D, Bunk O, Beraudi A, Ladisa M, Altamura D, Stea S, Baruffaldi F. Correlative light and scanning X-ray scattering microscopy of healthy and pathologic human bone sections. Sci Rep. 2012;2:435
- Tassani S, Matsopoulos GK, Baruffaldi F. 3D identification of trabecular bone fracture zone using an automatic image registration scheme: A validation study. J Biomech. 2012 Jul 26;45(11):2035-40
- Benasciutti E, Mariani E, Oliva L, Scolari M, Perilli E, Barras E, Milan E, Orfanelli U, Fazzalari NL, Campana L, Capobianco A, Otten L, Particelli F, Acha-Orbea H, Baruffaldi F, Faccio R, Sitia R, Reith W, Cenci S. MHC class II transactivator is an in vivo regulator of osteoclast differentiation and bone homeostasis co-opted from adaptive immunity. J Bone Miner Res. 2014 Feb;29(2):290-303
- Maffezzoni F, Maddalo M, Frara S, Mezzone M, Zorza I, Baruffaldi F, Doglietto F, Mazziotti G, Maroldi R, Giustina A. High-resolution-cone beam tomography analysis of bone microarchitecture in patients with acromegaly and radiological vertebral fractures. Endocrine. 2016 Nov;54(2):532-542. doi: 10.1007/s12020-016-1078-3. Epub 2016 Sep 6. PMID: 27601020
- Caravaggi P, Liverani E, Leardini A, Fortunato A, Belvedere C, Baruffaldi F, Fini M, Parrilli A, Mattioli-Belmonte M, Tomesani L, Pagani S. CoCr porous scaffolds manufactured via selective laser melting in orthopedics: Topographical, mechanical, and biological characterization. J Biomed Mater Res B Appl Biomater. 2019 Oct;107(7):2343-2353. doi: 10.1002/jbm.b.34328. Epub 2019 Jan 28. PMID: 30689288
- Zaghini A, Sarli G, Barboni C, Sanapo M, Pellegrino V, Diana A, Linta N, Rambaldi J, D'Apice MR, Murdocca M, Baleani M, Baruffaldi F, Fognani R, Mecca R, Festa A, Papparella S, Paciello O, Prisco F, Capanni C, Loi M, Schena E, Lattanzi G, Squarzoni S. Long term breeding of the Lmna G609G progeric mouse: Characterization of homozygous and heterozygous models. Exp Gerontol. 2020 Feb;130:110784. doi: 10.1016/j.exger.2019.110784. Epub 2019 Nov 30. PMID: 31794853
- Baruffaldi F, Mecca R, Stea S, Beraudi A, Bordini B, Amabile M, Sudanese A, Toni A. Squeaking and other noises in patients with ceramic-on-ceramic total hip arthroplasty. Hip Int. 2020 Jul;30(4):438-445. doi: 10.1177/1120700019864233. Epub 2019 Jul 21. PMID: 31328560.
The Experimental Biomechanics Unit is specialised in carrying out studies aimed at determining the mechanical performance of orthopaedic devices, orthopaedic scaffolds and surgical reconstructions of musculoskeletal system structures. Further studies investigate the mechanical behaviour of different components of the musculoskeletal system (tissues, organs, or multi-tissue units).
When possible, experimental tests are carried out according to relevant standards (ISO or ASTM standards). Also, the tests can be customised for special needs or even specifically designed to implement internal protocols simulating selected physiological loading scenarios. When aimed at the optimisation of surgical techniques, the experimental studies are designed in close collaboration with orthopaedic surgeons.
The collaboration with the In Silico Medicine Unit allows exploiting the synergy between experimental tests and numerical analyses, in order to establish prediction accuracy, i.e. how much the predictions are consistent with the experimental data, and then use the predictions of the validated models to reduce the number of experimental tests, thus increasing efficiency and reducing experimental costs.
The Register of Orthopaedic Prosthetic Implants (R.I.P.O) was established at the Rizzoli Orthopaedic Institutes in 1990. All the Orthopaedic Units in the Emilia-Romagna region (approx. 4 millions inhabitants) joined it from January 2000.
By December 31st, 2023 the Register collected data of nearly:
- 165.000 total hip-replacement, 22.100 hip revisions and 54.000 hemiarthoplasties;
- 148.000 knee replacements, 10.5000 knee revisions;
- 13.000 shoulder replacements.
More than 95% of surgeries performed in the Emilia-Romagna region are reported to RIPO, ensuring the reliability of the data analysis.
The collected variables are: side of surgery, reasons for revision hip, knee and shoulder prosthesis surgery, surgical procedure, complications during hospitalization, commercial type, reference code, and batch number of each single implanted component.
The end-point is the revision of even a single component of the prosthesis.
More than 100 different types of commercial hip prostheses and 90 knee prostheses are monitored to evaluate their outcomes.
The register co-operates with the orthopaedic surgeons and the regional authorities to define observational studies and to evaluate joint prostheses or innovative techniques. Moreover, the Register is able to identify in real-time the patients on whom a certain joint prosthesis was implanted. Those at risk of early failure based on recalls made by the Ministry of Health or manufacturers can thus be identified. At this point, orthopaedic surgeons may be in the position to promptly adopt all the necessary measures to defend the health of the patient.
The Register is funded by the Assessorship for Health and Social Politics of the Emilia-Romagna region.
The R.E.P.O. (Register of Orthopaedic Prosthetic Explants) management activity consists of the collection, classification, and analysis of medical devices explanted at the Rizzoli Orthopaedic Institute.
During 18 years of activity, more than 4.000 devices have been collected, mostly hip and knee prostheses. Devices involved in reported accidents are available to legal authorities, manufacturers and patients according to the internal procedure.
Publications of the referent of biological-and-clinical-research group
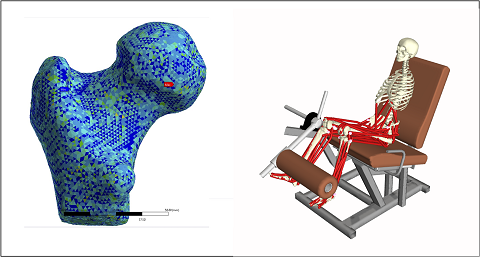
The research group focuses on In Silico Medicine, which involves the adoption of personalized computational models to support medical decisions and/or to assess the safety and efficacy of new medical products.
The activities mainly focus on applications related to the diagnosis and treatment of neuromusculoskeletal diseases, such as:
- Osteoarticular diseases: Finite element models are used to evaluate mechanical strength and risk of failure in bones (e.g., femur and vertebrae) under phisiological and/or phatological conditions (e.g., osteoporosis). Computational models are also used to predict and study implant failures (e.g., wear in hip and knee implants). Ongoing projects:
- BoneStrength (self-funded), aims to complete the development of the CT2S technology, extend its validation from the femur to the spine (including metastatic vertebrae), and qualify it with EMA
- IST4THR (self-funded), aims to develop in the long term a complete solution for in silico trials of total hip replacement medical devices
- COMPBIOMED (H2020), aims to scale up to HPC level in silico medicine applications, with a focus on MSK models
- Neuromuscular diseases: Computational models based on multibody dynamic systems are used to study the effect of musculoskeletal diseases (e.g., Parkinson and Sarcopenia) on motor control. Ongoing projects:
- MOBILISE-D (H2020), aims to qualify the average real-world walking speed, as measured with wearable sensors, as the main mobility biomarker in drug trials
- ForceLoss (RF2020), aims to develop, validate, and qualify subject-specific models of musculoskeletal dynamics as diagnostic and monitoring clinical tools.
Translation research is conducted on:
- Computational model verification and validation and regulatory science related to the adoption of these new technologies in clinical practice. Ongoing projects:
- STRITUVAD (H2020), aims to develop an in silico trial for tuberculosis therapies
- MOBILISE-D (H2020)
- Scalability and multiscale model orchestration. Ongoing projects:
- PRIMAGE (H2020), aims at developing a technology for the orchestration of in silico medicine models
- COMPBIOMED2 (H2020).
Also, different networking and community building initiatives are carried out to promote the wider adoption of in silico medicine. These include:
- InSilicoWorld, the first international online community for experts, researchers and professionals in the field of in silico medicine. The community hosts a discussion forum that collaboratively agreed on a set of Good Simulation Practices, besides other channels devoted to specific topics and project such as:
- Free Support Channel for computational medicine applications and scalability issues.
- Confidential forum where regulatory experts can freely discuss the best approaches to regulatory submission of In Silico Trial technologies for clinical practice
- International School on In Silico Trials, training initiative for decision-makers in medical industries, research hospitals, contract research organizations, regulatory agencies and notified bodies.
Staff











Contacts and Locations
Secretary's office
phone +39-051-6366864
fax +39-051-6366863
e-mail rosannaguida.franchi@ior.it
Rizzoli Orthopaedic Institute
Codivilla-Putti Research Centre
via di Barbiano, 1/10
40136 Bologna (Italy)



